Lifespan extension: separating fact from fiction
Thinking about longevity practically is a tricky affair. On the one hand, we have very little definitive knowledge about how to prolong your healthy years aside from the very obvious (exercise, don’t smoke, don’t be fat); on the other hand, by the time we have established that knowledge with much certainty, you may very well no longer be alive to take advantage of it. Coming up with actionable insights is therefore a complex exercise in scientific literacy and fuzzy evaluation of risk-reward tradeoffs. In this post, I would like to describe my own personal thought process and the conclusions to which I’ve come regarding my own “longevity stack.”
The purpose of this article is not so much to supply a laundry list of supplements but to instead provide the basis of a theoretical framework which the reader may apply to their individual practice. Generally speaking, I do not really like much of what circulates in the wild; people give too much credence to shoddy science but fail to assign enough trust to the best interventions. The most recent example which comes to mind is Bryan Johnson’s Blueprint, which may arguably do more harm than good, even if by its expansive nature does include one or two useful treatments.
The reader should note that this is not meant to be a comprehensive, perfectly reasoned review of all available evidence, which could easily stretch into the hundreds of pages depending on the level of detail demanded. Topics will be covered to the arbitrary level of depth which I personally judge to be appropriate; if felt inadequate, the reader may perform their own research, hopefully using the citations in this post as a useful springboard. Ultimately, if we want to apply this research to our own lives, we must accept some degree of inherent uncertainty, which the structure of this post intentionally reflects.
Disclaimer: This web site is provided for educational and informational purposes only and does not constitute providing medical advice or professional services. The information provided should not be used for diagnosing or treating a health problem or disease, and those seeking personal medical advice should consult with a licensed physician.
General approach
Let’s think a little bit about what the best piece of evidence for a given “anti-aging” treatment would be. In an ideal world, we would have evidence from multiple randomized interventional studies performed on large, representative, healthy human samples that independently demonstrate reproducible lifespan extension and delay of average onset for multiple age-related diseases. Obviously, if any such evidence existed for some compound, we would all know about it, and most of us would already be taking it; needless to say, there is no intervention whatsoever for which this level of evidence exists, even for interventions that “we all know” are good for us, like routine exercise.
We can weaken this requirement in two primary ways. First, we can accept evidence from nonhumans, allowing us to consider evidence generated from model organisms such as mice, C. elegans, and yeast. Such research may not translate cleanly to humans (if at all), but it stands to reason that there must exist mechanisms for extending lifespan which are conserved across species. Second, we can accept noninterventional human evidence, sacrificing some ability to determine causality but allowing us to combine compelling cross-sectional data with theoretical understanding of plausible anti-aging mechanisms. We will see that the most interesting therapeutics by and large tend to fall into one of these two categories.
Not all evidence is created equal. A core principle of my process of evaluation is that well over 50% of modern biological research is literally fake (fabricated from whole cloth), essentially fake (p-hacked or otherwise selectively presented), or so incompetently executed as to be pure noise. Additionally, entire fields of research are contaminated by severe financial conflicts of interest, and all academic research is governed by incentive structures hardly conducive to determination of absolute truth. Unfortunately, we only live once, and it is much easier to harm a complex physiological system than to improve it, so it seems prudent to apply a fairly conservative filter on the set of interventions that we choose to apply to our own bodies.
Additionally, concerns about drug-drug interactions, as well as the fact that known interventions likely target overlapping mechanisms (leading to rapidly diminishing returns if treatments are not carefully selected), are strong motivations to only consider the strongest bodies of evidence rather than adopting a “bucket list” approach. It is better to properly target a small number of known mechanisms in the safest ways possible using highly specific drugs than to take a hundred supplements, each with weak but pleiotropic effects and unknown absorption profiles, and hope that a good outcome is somehow achieved.
Overall, we are faced with a problem of considerable difficulty. Nevertheless, I believe that we now understand enough about human physiology and the effects of various drugs that a number of actionable recommendations can be produced from the mists of biomedical research. Let’s take a look.
Evidence from model organisms: the NIA ITP
Among model organisms, the most well-understood mammal is surely the mouse, and the gold standard for lifespan extension in mice is the National Institute of Aging’s Interventions Testing Program (ITP). This program tests a small number of drugs in large, well-controlled, multi-center studies to see if they extend the lifespan of genetically heterogeneous mice.
Several characteristics of the ITP are highly appealing. It uses a high sample size and experiments are well-powered to detect differences in lifespan; it actually measures total mouse lifespan, the most robust endpoint conceivable and one for which differences have the highest probability of translating to other species; it is a multi-center study performed in standardized conditions where control mice are not subject to artificially adverse environments; treatments are preregistered so results are not p-hacked; finally, the mice used are all from the standardized UM-HET3 four-way cross which do not have the genetic peculiarities of the vastly more common and severely inbred C57BL/6 strain. In short, it is a good experiment. On top of this, the ITP is an expensive program, so the set of compounds tested is selected based on the strength of preexisting evidence, meaning that there is typically a large body of supporting literature to corroborate positive results.
It is not uncommon for interventions to show lifespan extension in a lower-quality mouse study and to then fail to replicate in the ITP. The reasons why vary, but typically this is because a highly inbred strain is used in preliminary studies or because the mice are not housed properly (control group shows depressed lifespan); in both cases, the treatment is essentially fixing something that is deeply broken, rather than showing a benefit on top of a healthy baseline. For example, one might think that Dang et al. (2019) supplies intriguing evidence for pro-longevity effects of berberine, until one notices that the median lifespan in both control and treatment groups are below 700 days. We know that properly cared for C57BL/6 mice have a median lifespan of ~850 days, suggesting that the effects of berberine may not replicate in the context of a less dysfunctional colony.
If we want to choose treatments with the strongest evidence in model organisms, then, a logical place to look is at the ITP’s experimental results. In rough order of most to least replication within the ITP itself, the following compounds have been shown to extend both median and maximum lifespan: rapamycin, acarbose, canagliflozin, 17α-estradiol (males), and glycine (males only; weak effect), as well as various combinations thereof. (Metformin also extended lifespan, but only in combination with rapamycin; this is discussed further in a subsequent section.) The evidence for rapamycin is especially strong, with clear extension of lifespan by 20% or more across different starting ages and treatment regimes, suggesting that it is one of our strongest candidates for lifespan-extending treatments.
The case for rapamycin
The literature on rapamycin and its ability to extend lifespan at the correct dosages is extensive, so we will only briefly touch on its history here; much more detail is given in the magisterial review by Mannick and Lamming (2023). Briefly, genetic inhibition of the mTORC1 kinase was found more than two decades ago to extend the lifespan of multiple model organisms, a result which was then replicated many times over with rapamycin, an orally available inhibitor of mTORC1. At high doses, rapamycin is used as a potent immunosuppressant that prevents post-transplant rejection; however, evidence from animal models suggests that intermittent or low-dose treatment prolongs lifespan without exposure to undesirable side effects, such as total immunosuppression, hyperlipidemia, or hyperglycemia.
Rapamycin treatment seems to extend lifespan under a variety of different conditions. For example, Miller et al. (2014) and Miller et al. (2010) also report that the greatest rapamycin-dependent lifespan extension in the ITP is seen with treatment starting at 9 months of age in mice (equivalent to a 30-year-old human), with 23% extension in males and 26% in females:
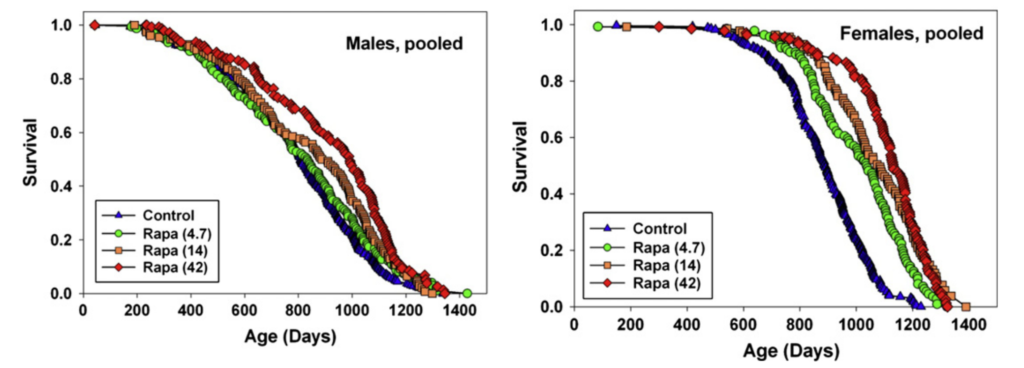
However, even when treatment was started in extremely old age (Harrison et al. (2009)), rapamycin extended lifespan by 9% in males and 14% in females:
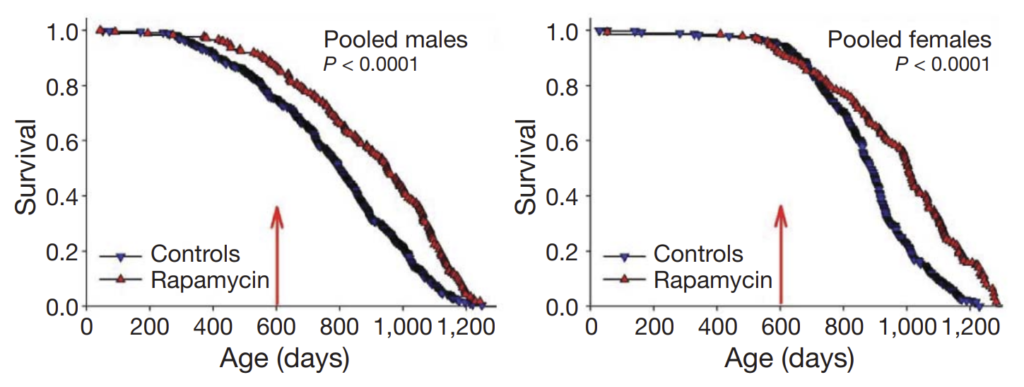
A starting age of 600 days in mice corresponds to approximately 60 years of age in humans, suggesting that a substantial benefit can be seen even if treatment is not started until one’s later years. (Note that the above graph demonstrates an artefactual separation of the survival curve for male mice even before the 600-day mark; the authors attribute this to unusual conditions at one or two specific study sites.)
Effects are also detected across varying treatment regimes, e.g. single dose vs. intermittent vs. continual administration (see for example Bitto et al. (2016) on transient usage in middle-aged mice), and rapamycin’s ability to extend lifespan is additive when combined with mechanistically distinct drugs like acarbose. Overall, the robustness of these results suggest that the beneficial effects of rapamycin have a relatively higher likelihood of translating to humans.
The fact that rapamycin is clinically used (at very high doses, achieving serum concentrations much greater than those of the mice in the studies above) as an immunosuppressant means that a priori, the most concerning potential side effect of rapamycin treatment, even at lower doses, is undesirable immune suppression. Curiously, however, limited evidence from human trials suggests that mTORC1 inhibition in the elderly can actually improve immune function:
- Mannick et al. (2014): “In elderly volunteers, as assessed by their response to influenza vaccination. [the mTOR inhibitor] RAD001 enhanced the response to the influenza vaccine by about 20% at doses that were relatively well tolerated.”
- Mannick et al. (2018): “A low-dose combination of a catalytic (BEZ235) plus an allosteric (RAD001) mTOR inhibitor that selectively inhibits target of rapamycin complex 1 (TORC1) downstream of mTOR was safe and was associated with a significant (P = 0.001) decrease in the rate of infections reported by elderly subjects for a year after study drug initiation. In addition, we observed an up-regulation of antiviral gene expression and an improvement in the response to influenza vaccination in this treatment group.”
- Mannick et al. (2021): “… in our second prespecified analysis, which included data from part 1 and part 2, we found a statistically significant reduction in the proportion of patients who had one or more laboratory-confirmed RTIs in the RTB101 10 mg once daily treatment group …”
While these studies did not use rapamycin itself, they supply compelling evidence that mTORC1 inhibition in the elderly can actually boost overall immune response (perhaps by reducing systemic inflammation that dampens the immune system), and indirectly suggest that when appropriately dosed, rapamycin should be relatively well tolerated in healthy adults.
A recent survey of off-label rapamycin usage by Kaeberlein et al. (2023) also suggests that the safety profile of rapamycin is fairly benign. While the data are purely observational and uncontrolled, it is still notable that the only adverse event that was significantly more prevalent in users of rapamycin was the formation of canker sores (mouth ulcerations), at a rate of 14.7% compared to 4.7% in the non-user group. While canker sores are a known side effect of sirolimus usage, they are relatively benign and self-resolving, and generally not considered to be a harbinger of broader, underlying autoimmune dysfunction in the same manner as other autoimmune disorders. Additionally, the self-reported weekly dosages of rapamycin are quite variable, and it is unknown to what extent the prevalence of canker sores varied with dosage.
Another way to consider plausible adverse side effects is to consider the beneficial effects of mTOR activation. Notably, it is well known (Watson and Baar (2014)) that the beneficial anabolic effects of exercise are mediated through mTOR signaling, and so a reasonable concern is that continued rapamycin usage would cause a reduction in muscular or skeletal mass. Physical frailty is a significant contributor to mortality and reduced quality of life in the elderly (Li et al. (2018)), so it is plausible that such effects, if present, could entirely counteract any beneficial effects of rapamycin. Thankfully, this does not seem to be the case: at low and intermittent doses, rapamycin in fact seems to protect against age-related loss of muscle and bone mass (see Wu et al. (2019), Orenduff et al. (2022), and many others).
Finally, a recent report by Artoni, Grützmacher, and Demetriades (2022) shows that rapamycin is a very “clean” drug, with very few off-target effects beyond inhibition of the mTORC complex at physiologically relevant doses. In general, off-target effects are a major source of specific toxicities; therefore, their absence suggests that there are very few “unknown unknowns” in rapamycin’s safety profile.
Looking beyond potential adverse effects, rapamycin also seems to demonstrate pleiotropic benefits in a number of disparate physiological systems in a manner consistent with a broad “anti-aging” effect:
- An et al. (2022) showed that rapamycin improved the oral health of aged mice, a result which should not be underestimated considering that periodontal disease is considered to be a hallmark of oral aging (An et al. (2017)) and a potential of infectious agents implicated in cognitive decline (Beydoun et al. (2020)).
- Altschuler et al. (2021) found that rapamycin treatment starting in late middle-age delayed age-associated hearing loss in genetically heterogeneous mice.
- Bitto et al. (2021) identified a protective effect of rapamycin against diet-induced obesity.
- Reports (unpublished) from resTORbio trial participants and off-label users suggest that commencement of low-dose rapamycin led to restoration of sexual function (morning erections in men and menstrual cycles in women). In the latter case, at least, such observations run complementary to the literature on the ability of rapamycin to slow ovarian aging in female mice.
The papers supplied here only constitute a small fraction of the available literature, all of which consistently points to the benefits of rapamycin treatment on multiple organ systems.
Importantly, the reader should take careful note here of the “flow” of our analysis. We began with the most challenging endpoint possible (extension of median and maximal lifespan) in a very rigorous, well-controlled study (the NIA ITP), checked that rapamycin performed well in that setting, then only afterward began considering individual, smaller, less well-controlled studies of alternate endpoints like muscle mass and hearing loss. The fact that we robustly observe a large absolute quantity of lifespan extension, which requires the benefits of rapamycin benefits to accrue systemically, significantly increases our credence in the weaker literature, which in the absence of that strong lifespan data is much more likely to be artifactual or p-hacked. In contrast, if we saw a number of different papers reporting benefits across multiple organ systems but eventually failed to notice any effect on lifespan in a rigorous study, that would suggest that the individual studies are themselves flawed, because an intervention that has a real effect on multiple aging phenotypes should (if real) be expected to extend total lifespan.
Overall, my own conclusion is that rapamycin has a risk-reward profile favorable enough that individuals, certainly those in middle or later age, and perhaps even those in early adulthood, should consider experimenting with a schedule of rapamycin supplementation. (Exact dosing details will be discussed in a later section.) Such a regimen is certainly not without risks, but it may also come with considerable benefits.
Comparing rapamycin to fasting
When trying to decide whether or not the risks of rapamycin are worth the potential upside, it’s useful to take a step back and consider the recent popularity of dietary interventions such as ketosis or intermittent fasting. A number of publications in the late 2010s (reviewed in de Cabo and Mattson (2019)) spurred substantial and enduring popular interest in time-restricted and/or compositionally-restricted dieting; either you have experimented with them yourself, or you are likely familiar with someone who has. Implicitly, people seem to accept that the risk-reward profiles of fasting, etc. are within the acceptable range.
However, these dietary interventions are themselves not particularly safe to begin with! A comprehensive review by Lee et al. (2021) notes that “anti-aging diets“ such as caloric restriction or intermittent fasting are often found to decrease lifespan in up to one-third of genetic backgrounds or to have directionally opposite effects on longevity in males vs. females. Severe dietary restrictions also come with their own specific problems: adherence is very challenging, alteration of dietary patterns impacts overall quality of life, and protein-deficient diets may even increase rather than decrease mortality in sufficiently aged populations (Levine et al. (2014)). Dieting also very obviously exposes people to risk of “adverse events” in the form of lower energy levels, dampened immune responses, grogginess, and mood alterations; however, because the nature of the intervention is behavioral rather than pharmaceutical, we do not typically consider these “risks” as properly as we should. Finally, part of the benefits of fasting are thought to originate from mTOR inhibition, suggesting that rapamycin administration could realize those beneficial effects with a much lower risk profile.
These lines of reasoning suggest that if we are willing to accept that intermittent fasting is potentially worth sustaining throughout your lifespan, then it is at least very plausible that taking rapamycin in some form, to cleanly achieve the benefits of transient mTOR inhibition without the numerous, “dirty” side effects of fasting, is a strictly superior option. Furthermore, conditional on already taking rapamycin, it is unclear that the marginal benefit of fasting is positive at all, i.e., taking into account the benefits of rapamycin itself, one should probably eat normally rather than bother with unusual dietary modifications.
The above discussion is inherently somewhat “sketchy” in nature, in the sense that there is little hard data to go off here, and we must rely on a good dose of analogical and first-principles reasoning. Unfortunately, when it comes to making practical decisions about lifespan extension, this sort of situation is the norm, and the wealth of data available to us on rapamycin treatment is by far the exception. One should note that there is a major exception to the above which comes to mind: in the case where an individual is prone to weight gain or already overweight, dietary interventions such as intermittent fasting or ketosis can make it easier to lose weight from a behavioral standpoint, and the health benefits of losing excess weight are likely so overwhelmingly great that the risk of adverse effects are inconsequential by comparison. In such a situation, then, one might consider taking rapamycin for its anti-obesogenic effects (Bitto et al. (2021)) as well as experimenting with different dietary patterns to see which one enables the most rapid weight loss.
Reduction of postprandial glucose spikes
Moving on from rapamycin, one immediately notices that several different promising interventions seem to fall under the broad class of pharmaceuticals that reduce postprandial (i.e., after eating a meal) glucose spikes:
- Acarbose, which inhibits α‐glucosidase and essentially reduces the rate at which glucose is released from complex polysaccharides, was found to increase the lifespan of male mice by over 15% and females by around 5% in the ITP (Harrison et al. (2014); Strong et al. (2016); Harrison et al. (2018)).
- Motivated by the strong results observed for acarbose, Miller et al. (2020) tested canagliflozin, an inhibitor of sodium glucose transporter 2 (SGLT2) that blocks renal reuptake and intestinal absorption of glucose, and found a 14% extension of median lifespan in male mice but no benefits for females.
- There are some indications that metformin, a poorly understood drug which is used as a first-line treatment for diabetes and is understood to lower blood glucose levels, might extend lifespan, although the evidence is murky; despite improving longevity of male mice in an earlier study, no pro-longevity effect of metformin was observed in the ITP (Martin-Montalvo et al. (2013), Strong et al. (2016)). Nevertheless, I still mention metformin here because (per Barzilai et al. (2016)), it is classically considered to be one of the first examples of a plausible “anti-aging” drug with metabolic effects that overlap with those of acarbose and canagliflozin.
Several observations point toward a common mechanism underlying these results from different drug regimens. All of these treatments are known to lower glucose levels in general, and they are typically used for this purpose to manage diabetes mellitus; however, the dosages of acarbose and canagliflozin used by the ITP are not known to lower hemoglobin A1C levels (a time-averaged measure of blood glucose levels over the past three months), suggesting that they act through dampening or smoothing glucose spikes rather than through reduction of total integrated glucose levels over time. The consistency of sexual dimorphism in the drugs’ effects on total longevity (if any effect is observed at all) is also suggestive of a a shared mechanistic basis.
Reduction of postprandial glucose spikes also seems to act through mechanisms which are largely distinct from mTORC inhibition, as shown in a recent study by Strong et al. (2022) who find additive beneficial effects from a combination of rapamycin and acarbose. Such treatments may therefore have high marginal value even for people who are already taking rapamycin.
However, from a purely practical perspective, acarbose and canagliflozin have a number of undesirable safety properties. For example, acarbose is known to induce diarrhea, and canagliflozin (along with comparable SGLT2 inhibitors) increases frequency of urination and genital tract infections. Anecdotally, subjects report that these side effects are not as severe when their diet composition includes fewer simple carbs, although this behavioral modification itself likely reduces the beneficial effects of these drugs via dampening postprandial glucose spikes, hence reducing the value proposition of the whole endeavor. Additionally, even a single incidence of untimely diarrhea or UTI contraction may easily lead to permanent cessation of drug treatment. Broadly speaking, I would find it difficult to recommend acarbose or SGLT2 inhibitors like canagliflozin for daily use.
That does not mean that this line of inquiry does not have insights to offer us! Thankfully, if it is in fact true that reduction of postprandial glucose spikes has a tremendous benefit to overall longevity, we can simply examine what the enormous literature on management of diabetes mellitus and glycemic load has to teach us:
- Yuan et al. (2014) showed that consumption of an additional 20 g dietary fiber in bread reduced peak postprandial glucose by >30% following consumption of a meal of pasta three hours after fiber intake, and even supplementation of psyllium husk or other forms of non-dietary fiber supplements once or twice a day is sufficient to reduce peak postprandial glucose (see review by Lambeau and McRorie Jr. (2017)).
- Exercising approximately half an hour after starting a meal is an effective way to reduce postprandial glucose spikes, with the degree of attenuation increasing with both intensity and duration of exercise (Erickson et al. (2017)). The exact parameters here will likely vary in realistic conditions, and realistically nobody is going to perfectly time their post-meal exercise every single day; however, it is hardly a burden to recommend a short bout of exercise (such as a stroll around the neighborhood) following large meals, which in any case is a pleasant lifestyle choice and should result in mild benefit even if not perfectly timed.
- Finally, obvious changes in dietary composition will reduce maximal postprandial glucose levels. For example, splitting up one large meal into multiple smaller meals or, alternatively, engaging in a practice of snacking throughout the day is almost guaranteed by definition to reduce peak postprandial glucose. Similarly, consuming more complex carbohydrates which take time to digest as opposed to, say, drinking soda or fruit juice will almost surely reduce peak postprandial glucose levels. In general, such “common-sensical” recommendations are consistent with the formalizations of glycemic index and glycemic load as found in the diabetes management literature (Vlachos et al. (2020)).
It is certainly possible that acarbose, canagliflozin, etc. also operate through alternate mechanisms which would not be captured by the above lifestyle or dietary interventions. However, the most parsimonious explanation for the results observed thus far is that reduction of peak postprandial glucose levels extends lifespan and slows the onset of age-related disease, especially (but not exclusively) in males. Given that fiber supplementation is extremely cheap and has essentially zero risk of adverse side effects, consumption of large amounts of soluble fiber at morning (as much as 10 g or more if tolerable) is nearly self-recommending. (Note that taking fiber alongside other medications is likely undesirable due to its ability to affect drug absorption; see e.g. González Canga et al. (2010).) Lifestyle changes such as post-meal exercise, slower mealtimes, and consumption of more complex carbohydrates also seem prudent, although ability to maintain such habits will vary per individual.
Speculation on the mechanism of 17α-estradiol
17α-estradiol, also known as a “non-feminizing” stereoisomer of 17β-estradiol, was first found to extend the median lifespan of male mice by 12% in the ITP by Harrison et al. (2014), work which was subsequently reproduced in a future ITP experiment by Harrison et al. (2020) with similar effect sizes:
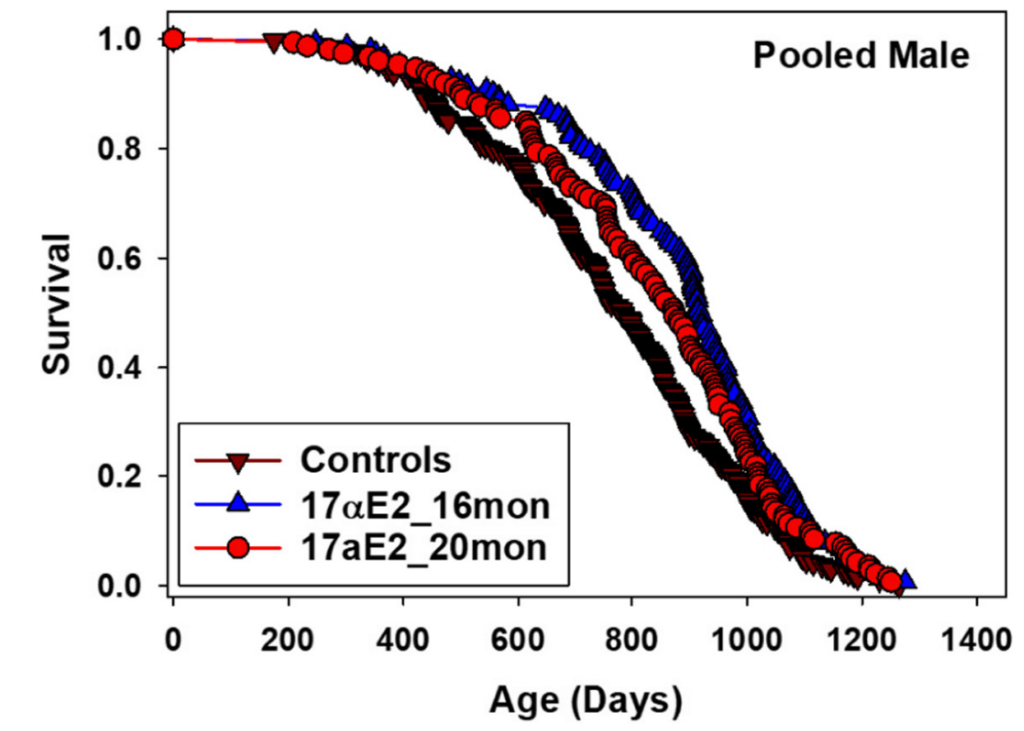
Curiously, while 17α-estradiol is bioavailable in both male and female mice, it only produces a pro-longevity effect in males, for reasons which remain unclear. Additionally, the greatest extension of median lifespan was observed when starting treatment at 16 months of age (19% improvement), while starting at 20 months of age yielded a considerably smaller benefit (11% improvement). It is therefore somewhat challenging to interpret these results into a clear recommendation for humans. Practical usage of 17α-estradiol is also frustrated by supply chain issues; it is unclear where to obtain stereochemically pure 17α-estradiol, how to compound it for oral ingestion, what the pharmacokinetics and pharmacodynamics are in humans, etc. Of the interventions which showed success in the NIA ITP, 17α-estradiol is definitely the most “annoying” to apply in practice.
Can we isolate the mechanistic basis of 17α-estradiol’s effect on lifespan and perhaps simplify the task before us with that information? A metabolomic study by Garratt et al. (2018) found that male mice treated with 17α-estradiol had higher levels of urea cycle intermediates and certain amino acids in the liver, and that these changes disappeared in a cohort of castrated male mice, suggesting that male-specific benefits are mediated through alterations to processes involving male gonadal hormones. This is potentially consistent with the observation that benefits of 17α-estradiol treatment decline after a certain age, as levels of androgen production are known to decline in elderly males. Additionally, the authors speculate that 17α-estradiol’s effects are mediated either through metabolites of 17α-estradiol such as conjugated estriols or, alternatively, through a direct inhibitory effect on 5α-reductase (5AR) activity, which blocks the conversion of testosterone into dihydrotestosterone (DHT).
If 17α-estradiol functions through direct association with estrogen receptors or through downstream metabolites such as conjugated estriol, I do not believe that there is sufficient information on safety, dosage, drug supply, etc. that lead to practical recommendations for men. However, one possibility does remain open to us, which is that at least some portion of the beneficial effects of 17α-estradiol treatment act through inhibition of 5α-reductase and reduction of DHT levels. Its ability to do so is sufficiently potent that it is commercially sold in a topical formulation to treat male pattern baldness through that exact mechanism; furthermore, acting through DHT reduction would be entirely consistent with a dependence on male gonadal hormones, because as males age, their testosterone levels naturally decrease, and since DHT is produced from testosterone, DHT levels also decline.
This observation suggests that we might be able to obtain some of the benefits of 17α-estradiol by taking highly specific 5α-reductase inhibitors such as finasteride or dutasteride, both of which are commonly used, orally available, inexpensive, and well tolerated drugs with uncommon side effects that generally reverse after cessation of treatment (Hirschburg et al. (2016)). Given the ease of obtaining finasteride as well as the general lifestyle benefits of preventing male pattern baldness for men, I believe it is easy enough to recommend finasteride (or dutasteride) on the basis of alopecia prevention alone; the addition of even potential benefit to longevity via a common mechanism with 17α-estradiol only further improves the risk-reward tradeoff of 5AR inhibition.
It is likely that in several years’ time, we will understand the mechanisms behind 17α-estradiol’s extension of murine lifespan sufficiently well to have a clearer idea of how to target the same pathways in humans. For now, my opinion is that the situation is murky enough that it is difficult for me to recommend self-administration of 17α-estradiol. However, we may at least hope to capture some of the benefits of 17α-estradiol via the far more well-understood finasteride or dutasteride.
Against supplementation of essential amino acids
The final and weakest successful intervention in the ITP was glycine, which, when supplemented to UM-HET3 at very high levels (8% of their diet), increased median lifespan of males and females by about 5% (Miller et al. (2019)). As glycine is a common amino acid and participates in a wide diversity of physiological processes, the underlying mechanism is not fully clear, but this result is consistent with a body of prior research which has found anti-inflammatory effects of glycine supplementation in rodents and humans alike. Coincidentally, Singh et al. (2023) recently reported that supplementation of taurine (a different amino acid) increased median lifespan of treated mice by over 10%, although such an effect is largely novel and the study was only performed in the highly inbred C57BL/6 strain. Unfortunately, taurine has not yet been tested in the ITP.
Do these results point at supplementation of certain amino acids as a potential way to extend lifespan of healthy adults? I do not believe so. Feeding mice 8% of their diet in a single amino acid (glycine) is a huge dosage which is completely impractical to reproduce in any human setting; the effect sizes seen are quite small and may very well attenuate even further when translated to humans; finally, to the extent that glycine supplementation extends lifespan by antagonizing methionine activation of the mTOR pathway (Kitada et al. (2020)), the same benefits are already achieved via rapamycin. In the case of taurine, there is but a single, unreplicated study, claiming an almost implausibly high effect size and what is, frankly, almost an excessively clean narrative of anti-aging effects in the absence of much background literature. Additionally, if we accept that glycine’s beneficial effects work through suppression of methionine-based mTOR signaling, we must also consequently accept that there is some generic risk that supplementation of the wrong amino acid (like methionine itself) could have an adverse, lifespan-reducing effect.
That brings us to the end of the (very short) list of interventions tested in the ITP which produced clear, robust extension of median and maximal lifespan of at least one sex! The reader, hopefully, can feel, as I do, the strength of supporting evidence declining quite precipitously as we move down the list. If even glycine, which is one of the very few successes of the ITP, does not seem worth using in humans, that suggests in a broad sense that the vast majority of supplements on, say, Bryan Johnson’s Blueprint are simply not beneficial.
It is important to emphasize here that the cutoff line I have chosen is relatively arbitrary. Certainly one could argue that glycine has some effect even when dosed at low levels to humans; one might even argue that the strength of the taurine study is sufficient even despite its lack of replication; one might then move down the list to increasingly unvalidated interventions, etc. However, again, from a precautionary perspective, in order to avoid the risk of wasting our time and money and introducing the potential for dangerous drug-drug interactions, I do not personally believe that it is worth considering interventions on the basis of model organism data beyond those listed above.
Epidemiological data on coffee consumption
Now that we have wrapped up our discussion of interventional data in model organisms, we can move to our second category of potential interventions, namely, treatments which appear to show robust benefits in noncausal human data. Unfortunately, there is no analogous NIA ITP in this setting, and hence no list of specific treatments we can work our way through.
Thankfully, there has been so little validated in this domain that it is quite straightforward to parse. A great deal of studies purporting to identify protective effects of specific foods have turned out to be hopelessly confounded by wealth. For example, red wine was famously believed to be protective against mortality at low levels of consumption; however, this story has been largely disproven at multiple levels:
- Larger, better-controlled studies have identified no protective levels of alcohol consumption (Zhao et al. (2023))
- The purported active ingredient of red wine, resveratrol has no pro-longevity effect in vertebrates (Hector et al. (2012))
- The sirtuin genes, thought to be the targets of resveratrol are not conserved longevity genes as originally believed (Brenner (2022))
- Resveratrol does not even target the sirtuins to begin with (Beher et al. (2009))
There is no longer any reason to believe that moderate alcohol consumption extends life or that any component of red wine exerts meaningfully beneficial physiological effects.
There is also much discussion about “Blue Zones” and the Mediterranean or Okinawan diets. Whether or not these diets are in fact good, there are far too many confounding factors (such as varying genetic backgrounds as well as the nonconstant nature of dietary practices over time) to say anything specific or to make precise recommendations, and I do not believe it is valuable to devote excessive attention to the literature in this area, as long as one makes some generic effort to “eat healthy.”
In actuality, I believe there is only one specific item of food (so setting aside nutritional components like fiber) which has met a sufficient standard of evidence that I would consider it somewhat likely to have protective, anti-mortality effects. This item is coffee, which shows a dose-dependent reduction in all-cause mortality across multiple large and well-characterized cohorts of diverse genetic backgrounds:
- Woodward and Tunstall-Pedoe (1999) studied a cohort of over 11k individuals from the Scottish Heart Health Study and identified a dose-dependent reduction of all-cause mortality in men and women up to 4 cups of coffee per day.
- Keefe et al. (2013) reviewed preexisting literature on coffee consumption, generally finding a dose-dependent relationship between cups of coffee per day and reduction of risk across multiple outcomes including all-cause mortality, coronary heart disease, and congestive heart failure up to 3-4 cups per day.
- Nordestgaard and Nordestgaard (2016) analyzed a cohort of over 100k White Danes and found that all-cause mortality risk was minimized at 4 cups of coffee per day, effectively plateauing at larger levels of consumption. Interestingly, Mendelian randomization was used with alleles known to be associated with caffeine intake to try to identify causality; at a genetic level, caffeine intake was not associated with lower risk of all-cause mortality, suggesting either deficiencies in the application of Mendelian randomization or a pro-longevity effect of coffee which is attributable to its non-caffeine components.
- Li et al. (2019) performed a meta-analysis of 21 cohort studies spanning over 10 million study participants and once again identified a nonlinear association between coffee consumption and all-cause mortality, with minimal risk attained at 3-4 cups of coffee per day. Interestingly, similar associations are found with both regular caffeinated coffee and decaffeinated coffee, which is consistent with the work of Nordestgaard and Nordestgaard (2016) and suggests a beneficial role for the non-caffeine components of coffee.
- Liu et al. (2022) report a prospective cohort analysis of UK Biobank participants showing that consumption of unsweetened or sugar-sweetened coffee, either regular or decaffeinated, again showed a dose-dependent reduction of all-cause mortality up to 3-4 cups of coffee per day. This relationship was attenuated when examining consumption.
Only a small sampling of the literature is given above, and the reader may verify that analogous results have been reported for a number of specific health outcomes beyond just all-cause mortality.
Despite efforts to control for socioeconomic background, wealth, ethnicity, weight, dietary composition, and so on, could it nevertheless remain the case that these results remain somehow confounded, and that coffee consumption actually has zero beneficial effect whatsoever? The possibility certainly exists, and in the absence of truly interventional data, it is very difficult to establish certainty about causality. However, given the other benefits of coffee consumption such as mental alertness and the low cost of coffee consumption, it seems prudent to recommend the consumption of 3-4 cups of coffee per day.
The exact mechanism behind coffee’s beneficial effects (to the extent that they exist) is not well understood, especially since caffeine does not seem to be the principal agent of interest here given that mortality risk is also reduced for consumers of decaffeinated coffee. Therefore, it is unclear if, at the margin, the benefits of coffee overlap with those already obtainable via mTORC inhibition or other mechanisms in previously recommended interventions. Curiously, the consumption of unsweetened coffee immediately prior to a meal appears to actually exacerbate postprandial spikes in blood glucose (Louie et al. (2008)), perhaps motivating an approach where one restricts coffee consumption to interludes between mealtimes or in conjunction with an intentionally light breakfast.
Finally, it’s worth noting that residual confounding can influence effect sizes in both directions. Specifically, although coffee consumption seems to have a “U-shaped” dose-response curve with respect to longevity, with all-cause mortality risk seemingly increasing after 4-5 cups of coffee per day, one should consider the possibility that it is so unusual to drink >5 cups of coffee per day that any person who does so is likely subject to abnormal stressors which are inadequately captured in the statistical analysis. Were this to be the case, this could mean that the health benefits of coffee might even be underestimated, with the optimal dosage potentially far exceeding 5 cups per day. Of course, we are now well within the realm of pure speculation, but if we are concerned about socioeconomic confounders that lead us to overestimate the benefits of coffee consumption, we may at the very least acknowledge the possibility that confounders in the other direction may very plausibly exist.
Regular blood donation likely has systemic benefits
Beyond dietary interventions, one may also wonder what lifestyle habits (beyond the obvious ones like exercise) may reduce mortality risk. I believe that frequent blood donation is a compelling candidate here, because it combines weak (but suggestive) cross-sectional data with interventional data in mice and a solid theoretical basis for positive effects.
A study by Ullum et al. (2015) first suggested that blood donation could exert a pro-longevity effect: controlling for the “healthy donor effect” (where people with better underlying health status are more likely to donate blood), they nevertheless identified a 7.5% reduction in all-cause mortality risk per annual donation in a large cohort of nearly 10 million Scandinavians.
It is, of course, difficult to simply control for what may be deeply pervasive selection effects that elude straightforward quantification, and blood donation carries with it its own risks and adverse effects, ranging from simple skin bruising to temporary dampening of the immune system due to systemic stress. However, two supplemental observations work to considerably strengthen, in my opinion, the rationale behind frequent blood donation.
The first observation is that excessively high iron levels are associated with excess mortality and moderately blocking iron absorption in model organisms is known to prolong longevity (Mangan (2021)). While low hemoglobin levels and idiopathic anemia become increasingly prevalent with aging, and individuals should take care to ensure that their iron levels remain above anemic levels, the evidence weakly suggests that the optimal range for longevity is at the lower half of what is typically considered the “normal range.” Indeed, to the extent that consumption of red meat elevates mortality risk, it may partially do so through excessive elevation of iron levels (Etemadi et al. (2017)). Blood donation, of course, directly removes iron from the body, and preliminary evidence in mice from Liu et al. (2022) suggests that blood donation ameliorates skin inflammation in old age via clearance of iron deposits.
The second observation, which constitutes a fully independent line of evidence and hence lends substantial marginal credence to the overall thesis of beneficial blood donations, is the study of heterochronic parabiosis in mice, in which the circulatory systems of an old and young mouse are surgically connected. Despite being a deeply invasive and traumatic procedure, considerable physiological benefits and rejuvenation of aged tissues effect have been observed for the older mouse (Conboy et al. (2013); Eggel and Wyss-Coray (2014); Ma et al. (2022)). Originally, it was believed that these benefits came from connecting the circulatory system of the old mouse to the organs of the young mouse, essentially allowing the old mouse to benefit from a set of younger organs; however, work by Rebo et al. (2016) showed that even a single infusion of old blood exerted severe deleterious effects on a young mouse’s physiology, suggesting that the benefits of heterochronic parabiosis are partially, and perhaps almost entirely, attributable to dilution of detrimental inflammatory factors in the circulatory system of the old mouse with “clean“ blood from its younger partner. We know that systemic inflammation is a hallmark of aging and that elderly human blood does contain severely elevated levels of a broad range of inflammatory factors, which, when isolated, have almost strictly negative effects, so this model of heterochronic parabiosis is fully consistent with prior understanding, and predicts that blood donation should have a beneficial effect via depletion of undesirable circulating factors.
Because the blood donor population is so heavily selected, it is unclear that we will obtain clear epidemiological evidence on the benefits (or lack thereof) of blood donation in the coming years. However, in this particular case, I believe that the sum totality of the evidence makes the risk-reward profile of regular blood donation at a high frequency (perhaps monthly or bimonthly) quite attractive.
The jury is still out on saunas
Beyond blood donation, there has been a slowly growing literature on the benefits of spending more time (up to 3-4 sessions a week) in hot saunas: starting with Laukkanen et al. (2015) and extending all the way to Kunutsor et al. (2022) and beyond, a series of papers claim to identify dose-dependent reduction of cardiovascular and all-cause mortality risk attributable to sauna usage. While sauna usage is quite enjoyable, I am skeptical of these claims, and I present them here as an example of the “boundary” of my reasoning about weak epidemiological evidence.
One obvious rejoinder is that these studies are confounded by the fact that regular sauna-goers may be wealthier, exercise harder, etc. in ways that are difficult to perfectly control for, meaning that the results are simply detecting residual confounding. Surely this could not be the case, though, across so many different publications? Well, as far as I can tell, all of these papers study the exact same cohort data (from the Finnish Kuopio Ischemic Heart Disease Risk Factor Study) and involve overlapping researchers, so it would in actuality be unsurprising if the same flaws in the data were present across all these separate analyses. It is true that Finland is a relatively egalitarian society, but even so, this type of result standing alone appears quite suspect, although it would be interesting to see if it persisted after correction for potential genetic confounding using polygenic scores.
There is admittedly a rather weak theoretical basis for heat shocks playing a role in lifespan extension. Notably, the heat shock proteins (HSPs), so named for their role in response to stressors such as heat shock, are known to be a highly conserved pathway and sometimes thought of as potential mediators of hormetic lifespan extension following mild heat shocks, although actual evidence of such an effect in model organisms (Lagisz et al. (2013)). On the one hand, this literature dates back many decades, and it does seem likely that the bulk of the research on HSPs is legitimate; on the other hand, the failure to identify a clean interventional effect here after so many years is not a promising sign.
Very speculatively, one could argue (as Peter Attia does) that transient elevation of heart rate mimics some portion of the effects of cardiovascular exercise. However, sauna usage is quite inconvenient and likely only incompletely mimics exercise if it does so at all, whereas the research on the systemic and far-ranging benefits of even light, time-limited cardiovascular exercise is strong and incontrovertible; hence, again taking into mind a marginal perspective, it seems more sensible to recommend some modest amount of cardiovascular exercise rather than sauna sessions four times a week.
There at least seems to be little reason to avoid saunas, and because I quite enjoy using them, I will likely still keep the pro-longevity results for sauna usage in mind as I relax in the sauna after a hard gym session, for emotional gratification if nothing else. However, at a purely intellectual level, it is difficult to say that the evidence here passes even a minimal standard of basic quality and plausibility, let alone a considered risk-reward analysis on top of a base of more well-validated lifestyle changes like regular cardiovascular exercise or resistance training.
Anti-recommendations: NAD+ precursors, senolytics, polyphenols
Now that we have largely finished discussing positive recommendations generated from the basis of model organism or human data, I want to at least briefly discuss some negative recommendations. In general, there are of course innumerable poorly supported interventions which one could spend a long while dismissing; therefore I will limit myself here only to those which I feel are the most commonly brought up as potential interventions of personal interest.
There is an almost overwhelming amount of popular interest around NAD precursors. The basic idea is that levels of NAD+ or NADH (respectively the oxidized and reduced forms of nicotinamide adenine dinucleotide, together referred to generically as NAD), which are important cofactors in innumerable metabolic reactions, decline with age, and so increasing intracellular NAD concentrations may have an “anti-aging” effect. This can be done either via intravenous administration of NAD, which is of course cumbersome in a non-medical setting, or via supplementation of NAD precursors, which are claimed to eventually increase physiological NAD levels. The two most popular NAD precursors are nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), both of which are heavily promoted by research out of the Sinclair Lab and commercially sold by companies such as Elysium in which leading NAD researchers tend to have financial stakes.
Immediately, the presence of such strong financial conflicts of interest should make one suspicious of the entire field. On top of that, frequently cited research is generally of a very low quality; for example, in a study of the efficacy and safety of NMN in humans by Yi et al. (2023), nonsensical results were reported via their measure of “blood biological age” according to which the control group aged 5.6 years in the span of 60 days. (More problems are described in a detailed post by Peter Attia.) When studies in model organisms are performed with greater care, such as in NIA’s ITP, only null results on lifespan are identified. Finally, the safety profile of NAD precursor supplementation is far from ideal; for example, Maric et al. (2023) identified a role for NR supplementation in worsening cancer prevalence and metastasis, and unpublished work by Saleh et al. claims that NMN actually exacerbates inflammation in the kidneys of aged mice.
Given the fact that negative effects of NR or NMN treatment have already been identified, alongside the general shadiness of the entire field and its contamination by commingled academic and commercial interests, I would stay away from the entire NAD field with a 1,000-foot pole, and if I had any friends considering taking any NAD-related supplement I would do my best to convince them otherwise. I view the vast majority of the research here as at best an abdication of scientific duty, at worst a moral crime against fellow man.
Senolytics form a different sort of negative recommendation altogether, in that while the underlying science is reasonably strong, there are simply no known options for direct and targeted senolysis in humans. To briefly review: cellular senescence is a state of irreversible growth arrest, which can be triggered either by replicative exhaustion of telomeres or by various stressors such as irradiation or the upregulation of certain cancer-related genes, and while senescent cells are typically cleared by the immune system, they are known to persist in increasing degree with age and to secrete a complex set of immune factors, known as the senescence-associated secretory phenotype (SASP), which both results in systemic inflammation and aids continued immune evasion of the culprit cells (van Deursen (2014)). Several years ago, researchers observed that ablation of all senescent cells in mice genetically engineered with a highly specific “killswitch” increased lifespan and resulted in the rejuvenation of multiple organ systems, leading to rapid interest in identification of pharmaceutical interventions (“senolytics”) which could selectively induce senecent cell death (van Deursen (2019)).
Sadly, even now, the state of senolytic therapies remains dismal. As recently reviewed by Chaib et al. (2022), most research on chemical senolysis focuses on repurposing of cancer drugs with highly specific toxicity or derivatives thereof (navitoclax, nutlin-3a, UBX0101), treatment with extremely high doses of chemically “dirty” natural compounds with questionable absorption and stability (fisetin), or tenuous combinations of the two (dasatinib + quercetin). While these compounds ultimately do work in some sense, and at the very least reproducibly kill senescent cells in vitro, they are highly unsuitable for human administration due to a combination of factors such as both on-target and off-target toxicity, poor metabolism, insufficient systemic distribution, etc. It is little surprise that the state of the field as regards these “popular” compounds remains much the same as it was several years ago, i.e., mostly a failure. Attempts to improve delivery of inferior compounds like fisetin with, for example, lipid nanoparticles are also quite likely to be doomed to failure (one cannot easily put lipstick on a pig), in addition to being impossible to actually use at home.
Less well-characterized senolytics with one-off publications are unfortunately also likely to be duds. In my own experience, the FOXO4-DRI peptide reported by Baar et al. (2017) never worked. Similarly, neither of the circadian modulators SR9009 or SR9011 reported by Sulli et al. (2018) showed even the smallest inkling of senolytic activity across a variety of experimental conditions. Etc. While there is nothing wrong in principle with the idea of selective ablation of senolytic cells, and one day we will likely have potent, safe, and orally available senolytic drugs available to us, the state of the field is simply not there, and I do not believe there are any actionable insights that one can take away from the totality of research on senolytic therapies.
Finally, the SASP suppression associated with rapamycin treatment identified by Wang et al. (2017) makes the marginal cost vs. benefit of “direct” senolysis even worse. The SASP is not just a source of inflammatory factors radiating outward into the rest of the body but in fact an important contributor to a senescent cell’s ability to evade clearance by the immune system (Muñoz et al. (2019)). To the extent that rapamycin suppresses the SASP, then, it may very well even result in clearance of accumulated senescent cells by the immune system, which is still able to recognize senescent cells via the presence of characteristic surface proteins. Therefore, for someone who is already taking rapamycin, the marginal benefit of experimenting with highly “dirty,” poorly validated senolytic therapies is tremendously low.
The point about poor chemical characteristics of natural compounds such as quercetin or fisetin also applies more generally to plant polyphenols, flavonols, and so on. Consider, for example, the absurdly long list of supplements in Bryan Johnson’s Blueprint:

The presence of turmeric on this list is emblematic of the problems with including natural products on the basis of low-quality evidence (which may exist in abundance) and ignoring any basic standard of evidence for efficacy, absorption, distribution, metabolism, excretion, and so on. Turmeric, as well as its most well-known chemical constituent curcumin, have been studied in thousands of publications, named in hundreds of patents, and tested in humans across over a hundred clinical trials. Yet there is no plausible reason from a chemical perspective why curcumin could ever produce any of the miraculous effects attributed to it in breathless paper after paper; its bioavailability is under 1% and has a half-life of several minutes, and Nelson et al. (2017) describe, with considerable care, the fact that turmeric or curcumin have never had a credibly positive result in a clinical trial and that its positive activity in in vitro assays are most likely all false positives attributable to its highly promiscuous chemical structure.
It is well known by medicinal chemists (Beall (2014)) that many categories of natural compounds, such as polyhydroxylated natural phytochemicals, polyphenols, flavonoids, and so on are highly reactive in ways that confound assay results; some are naturally fluorescent or colorful, others have a propensity to trap catalysts used in various assays, redox cyclers may naturally produce hydrogen peroxide in common assay conditions, and so on. All of these flaws continue to lead to persistent identification of false positive results, which to this day continue to get published nonstop (simply search “curcumin” on Google Scholar to verify this yourself). As such, when one sees a natural compound or supplement derived from something that intuitively “sounds” healthy, like a common Indian spice (turmeric), a bitter vegetable (“BroccoMax”), or a pungent herb (garlic extract), the overwhelming bulk of the probability lies on the entirety of the supporting literature being wholly artefactual and the supplementation itself being a total waste of time and money. And even if there is some real effect being measured, natural products are often as far from optimized, orally available drugs as you can get; as with curcumin, whatever you ingest is likely to be barely absorbed and almost immediately metabolized, to say nothing of whether or not the active compound gets properly distributed to the physiological subsystem of interest.
Cosmetic preservation of skin quality
Let’s close on a somewhat lighter note: are there straightforward ways to preserve the cosmetic quality of your skin? The connection to lifespan is, of course, tenuous at best; however, it would be difficult to imagine someone interested in preservation of their youth who does not have at least some interest in preventing some of the most visible ravages of age. After all:
“Beauty is truth, truth beauty,—that is all
Ye know on earth, and all ye need to know.”
(Keats, Ode to a Grecian Urn, 1819.)
The most obvious candidate for preventing photoaging is sunscreen, and daily usage of SPF > 30 sunscreen is strongly recommended for this purpose (Guan et al. (2021)). I believe that it is not necessary to spend much time here establishing sunscreen usage as effective in the prevention of photoaging and melanoma. However, there is an interesting, longevity-related point to be made here, which is perhaps underappreciated by the public: sun avoidance specifically is associated with an elevated risk of all-cause mortality even after controlling for exercise habits (Lindqvist et al. (2014)). The exact mechanisms underlying this association, if it is in fact non-artefactual at all, remain unclear; however, one possibility is that sun avoidance is detrimental to health through reduction of basal levels of vitamin D production.
If this is true (admittedly there is little to prove or disprove this chain either way), then given the low levels of sun exposure experienced by modern white-collar professionals, sunscreen use could plausibly reduce vitamin D levels even further to clearly suboptimal harmful levels. The data here are relatively scarce, but a review by Neale et al. (2019) found that standardized experimental studies consistently identified a reduction in vitamin D production in response to artificial ultraviolet radiation following sunscreen application, suggesting that sunscreen usage (especially at high SPFs) does in fact reduce physiological vitamin D levels. The authors also comment that randomized field trials, in contrast, did not identify such an effect; however, such trials tended to use low SPF sunscreens (~ SPF 16), and one can imagine that their results were plagued by extremely high variability in participant compliance and behavior.
My personal approach, which is mostly based on my vague synthesis of weak evidence and general biological intuition, is to apply sunscreen on my face every morning and to apply sunscreen on all exposed limbs if going outside for a prolonged duration on a sunny day. (Readers who dislike the greasy, staining qualities of American sunscreens are advised to try Asian brands, which are not subject to the archaic sunscreen ingredient regulations imposed by the FDA.) I also try to get some exposure to natural sunlight every day. Finally, I take a 10k IU vitamin D supplement orally every night; the amount is somewhat arbitrarily chosen, but vitamin D is quite well tolerated, and given that I personally have an indoors-heavy lifestyle, I believe that the balance of risk versus reward leans toward over- rather than under-supplementation.
Moving on to consider purely cosmetic treatments: the use of topical retinoid creams has long been understood to help in the preservation or restoration of skin quality, especially in the presence of preexisting photoaging (Mukherjee et al. (2006)). They are now considered to have prophylatic effects against loss of, inter alia, epidermal quality and collagen degradation (Zasada and Budzisz (2019)). As retinoid creams are well understood as a standard component of first-line dermatological treatment, I will not spend too much time describing them here; however, of the two options typically recommended (tretinoin 0.025% cream and adapalene 0.1% gel), I have a preference for adapelene 0.1% gel (sold as Differin in the United States), because adapalene 0.1% seems to be just as efficacious as tretinoin 0.025% while being much less irritating to patients (Dunlap et al. (1998), Galvin et al. (1998)).
Finally, for those willing to seek treatment from a dermatologist, microneedling is a relatively inexpensive and noninvasive procedure that “produces substantial clinical improvement of scars, striae, and rhytides with expedient recovery and limited side effects” (Alster and Graham (2017)). I would caution the reader that one should avoid the temptation to perform “at-home” microneedling with an inexpensive dermaroller; while the mechanisms are not fully understood, it is believed that improperly performed microneedling will obliquely puncture the skin to an insufficient depth, leading to more rather than less scarring, in contrast to professional microneedling in which skin is punctured at an exact right angle to the proper depth.
Tying everything together into a treatment plan
We have covered quite a bit of ground in this article. However, what is our final set of concrete recommendations? To summarize the discussion above, here is what I would concretely propose for an “anti-aging” regimen on the basis of presently existing evidence:
- Medications. Take rapamycin, either 2-6 mg once per week or 20 mg once per month. (Dosing will be analyzed further in a subsequent Milky Eggs post.) If male, take 1 mg finasteride every night.
- Other supplements. Take 10 g of fiber every morning and 10k IU of Vitamin D every evening.
- Diet. Consume a good balance of macronutrients with preference for complex carbohydrates and fibrous foods and preference against simple sugars. Drink four cups of coffee per day, ideally in the morning or between meals. Take short walks after eating.
- Weight loss. If overweight or obese, lose weight with GLP-1 agonists like semaglutide and behavioral changes to dietary patterns such as an intermittent fasting routine.
- Exercise. Maintain a moderate workout schedule that includes both resistance training and cardiovascular exercise.
- Skincare. Apply sunscreen every morning; clindamycin (if acne is a concern), adapalene 0.1% cream, and moisturizer every night. Undergo microneedling treatments under supervision of a skilled dermatologist.
- Blood donation. Donate blood at least several times a year, increasing frequency if hemoglobin levels are in the upper range of normal and decreasing frequency if hemoglobin threaten to decrease to anemic levels.
I have included some basic advice which I have not bothered to carefully justify; hopefully the reader does not need convincing that obesity is bad or that exercise is good. You may notice that this does not seem like an especially radical set of recommendations. It is indeed not, and that is where I feel the best balance of risk and reward lies! However, even if you disagree with my specific conclusions, I hope that my method of thinking as exemplified in this post may prove useful to your own research.
In short, most of the novelty in this post really comes from several simple observations. First, rapamycin is probably both safe and beneficial. Second, the pro-longevity effects of diabetes management drugs likely comes from reducing postprandial glucose spikes, which is more easily achieved through fiber supplementation and post-meal walks. Third, men should take finasteride and (nearly) everyone should donate blood regularly. Fourth, you should make an effort to ignore the enormous amounts of nonsense which circulate online, such as breathless discussions about NAD precursors, berberine, etc.
There are several directions of future inquiry which I would like to explore in a follow-up post:
- For someone starting young, what is the ideal rapamycin dosing schedule? Should they prioritize frequent low doses, such as 3 mg per week, or infrequent high doses, such as 20 mg per month? The first scenario allows for more frequent transient mTOR inhibition and avoids acute danger from the immunosuppression of higher dosages; the second scenario may, however, be what is necessary to achieve CNS concentrations equivalent to those seen in ITP mouse studies.
- For someone in reasonably good health with a moderately good diet, what is the risk-reward profile of chronically taking a statin (e.g. atorvastatin) to reduce non-HDL cholesterol levels beyond the “normal” range?
- What about other supplements which come up frequently in online discussion like zinc, magnesium, and fish oil? Even if they fail to increase the longevity of laboratory mice, do they correct deficiencies that are common in the modern lifestyle or otherwise have compelling evidence that support their supplementation? (In general, I lean towards no, but it would be worth revisiting the literature one last time.)
While intriguing, we will leave these questions to rest until a future data.
The paucity of clearly pro-longevity treatments should also not be taken as a disheartening sign, especially for those who are young. Much promise lies only just beyond the horizon: understanding the mechanistic basis of 17α-estradiol; developing therapies based on α-klotho; discovery of inexpensive, orally available, and safe inhibitors of inflammasomes like NLRP3; perhaps even the development of safe and efficacious senolytic drugs available to the public at large. We do not need to have all the answers in hand right now; instead, we need only do what we can and patiently wait for the inevitable rise of the (bio)technological tide.
Contact
For personal inquiries, I can be reached through email at milkyeggs2@gmail.com or on Twitter at @0xfbifemboy.
July 9th, 2023 at 12:18 pm
There’s a bit of tension between the suggestions here: Usually, blood donation places won’t take your blood if you’ve taken finasteride in the past month.
July 9th, 2023 at 3:27 pm
The Mendelian randomization data on genetically lower LDL levels correlating with longevity and not having any cognitive side effects like high rates of dementia, as well as heart disease being the leading cause of death in high income countries makes some form of LDL lowering a good idea. The downside of statins is that they do increase the risk of diabetes slightly, but you can monitor A1C levels and just stop it if it turned up or use medications or supplements with alternative mechanisms, like Ezetimibe.
July 9th, 2023 at 9:38 pm
@Will: That is a pretty compelling argument. Good Mendelian randomization results are always nice. I might edit in a statin recommendation once I read up a bit more about them.
July 9th, 2023 at 6:11 pm
banger
July 9th, 2023 at 8:18 pm
Is there any reason that females should be more cautious with rapamycin than males, i.e decreased fertility or unhealthy future offspring?
July 10th, 2023 at 6:36 pm
It is bananas how few supplements have quality evidence for benefit.
I always wonder what ways you could get 90% of the results with less $/effort and more simplicity, the only way I can think of is to:
– Intermittent fast/snack on fruit most of the day, active and in the sun. The potassium in fruit replaces some insulin and blood sugar is well-controlled.
– Eating nose-to-tail and bone-broth (glycine) with carbs in there. Enough protein to be adequate but not too much to avoid spiking blood sugar. The hazards of fasting would be avoided by keeping stress down via lifestyle and small snacks.
The main benefit of your stack is that it’s more resilient to a stressful and indoor lifestyle than the above.
July 23rd, 2023 at 8:19 pm
Finasteride can hinder beard growth, though, so maybe the blank recommendation should be just for men who prefer to stay clean shaven.
July 24th, 2023 at 2:31 pm
Also recommended is checking your iron levels prior to donating blood (ideally including Ferritin, as serum iron counter-intuitively spikes in severe chronic deficiency). Donation centers will only check very basic measures to ensure you’re not obviously anemic, but this will not catch chronic iron issues preceding iron deficiency anemia. Ask me how I know—I donated whole blood (double, to boot) several times last year, unaware of an existing long term deficiency, which then accelerated my iron stores dropping below the threshold where physiologic effects began (anemia, thyroid hormones going out of whack, etc).
July 24th, 2023 at 4:31 pm
4 cups of coffee per day? that is a lot and most cannot do it as will affect sleep which I assume you know
Specifying an intake of Vitamin D is not wise without saying what a blood range level should be. Different people will absorb, process and convert the supplement in to varying amounts in the body. There is no good research to suggest that very high levels of Vitamin D, above say 40 are beneficial. Vitamin D more and more is looking like Vitamin C; min dose needed.
!0G of fiber in what form? Psyllium husk, via Metamuceil?
July 25th, 2023 at 4:21 am
@noimporta my local Red Cross in fact told me that finasteride was a three month deferral due to (IIRC) risk of birth defects if the donated blood is administered to pregnant women. I wonder if it is possible to hack around this by paying to have one’s blood drawn for one’s own (autologous) possible future use.
July 26th, 2023 at 10:03 pm
Rapamycin sounds promising, and a lot of your other recommendations are solid AFAIK. However, given that finasteride can cause fetal birth defects and irreversible sexual dysfunction, I’m surprised you recommended it. I’d encourage anyone curious about *any* of these approaches to investigate thoroughly before jumping in.
July 26th, 2023 at 10:14 pm
Regarding “Exercising approximately half an hour after starting a meal” – a ten minute walk may suffice, at least according to Rhino.
Shorts version: https://www.youtube.com/shorts/rgcwQlmiUIs
Extended version: https://www.youtube.com/watch?v=xyrmMjxHzPE
July 30th, 2023 at 3:51 pm
Hi,
just in case you didn’t know: your website certificate has expired. Or at least Firefox thinks it has.
Dave.
August 15th, 2023 at 1:57 pm
Thanks for this great article!
Besides smoothing glucose spikes, could Acarbose enhance longevity by regulating the imbalance of the microbiome flora? See: https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-019-1494-7
What about GLP1 agonists? Although not yet tested in the ITP, they seem to have systemic effects besides blood sugar management and weight loss, e.g.: https://cureparkinsons.org.uk/2021/11/the-exenatide-trial
For NR, the results of this trial will be interesting to follow: https://beta.clinicaltrials.gov/study/NCT03568968
Mice don’t commit suicide, so what about interventions to improve mental health? (Light therapy, creatine, lithium, psychedelics, CBT, exercise, etc.)
August 16th, 2023 at 8:36 am
One more thing on Acarbose: “only” 14% of patients report diarrhea. Effects are dose-related so starting with a low dose is quite safe. What’s more, side effects decrease over time, even on constant dosing: https://linkinghub.elsevier.com/retrieve/pii/S0002934397002520
As a matter of comparison, semaglutide that you mention for weight loss has similar side effects:
https://www.nature.com/articles/s41591-022-02026-4
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9631444/
September 10th, 2023 at 5:16 pm
great read, loved it. a bit surprised about the short treatment of metformin, so there’s just not that much to it. looking forward to your follow up posts, especially the one about statins.